Development And Pulmonary Absorption Study of Dry Powder Inhalers Using Aerosol Chamber in Mice
Keywords:
- Dry Powder Inhalers (DPIs),Pulmonary drug delivery, Formulation strategies, Aerodynamic optimization ,Preclinical evaluation
Abstract
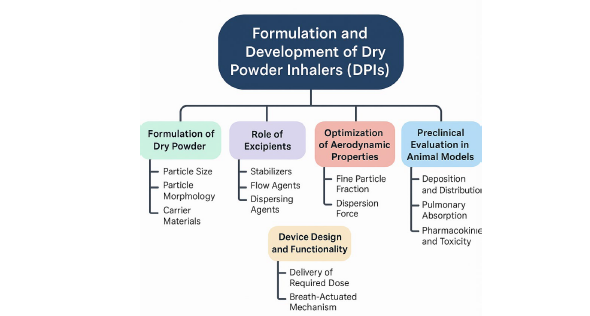
DPI (Dry Powder Inhalers) have become an attractive system for targeted pulmonary delivery of drugs due to the localized activity, quick onset of therapeutic activity, and low levels of exposure systemically. This study is a review of the formulation strategies, aerodynamic optimization, and preclinical assessment of DPIs in the animal models particularly mice. The use of such devices as aerosol exposure chambers in inhalation studies has allowed for the consistent and accurate delivery of drugs using the natural process of breathing and, hence contributing to the improvement of preclinical evaluations’ reliability. The review also highlights the importance of assessing pulmonary absorption, deposition efficiency, and pharmacokinetic profiles in animal models in order to back safe and effective designs of DPI formulations. Findings from literature have shown that DPIs, formulated and administered correctly, can provide effective lung deposition and absorption, which sets a formidable preliminary stone for subsequent medical applications based on respiratory care. The study further notes gaps in research and the future directions of improving DPI development and evaluation using animal-based models.